Soft Matter at Interfaces
Soft matter at interfaces is present in biological and industrial systems. For intense, surfactant molecules or polymer colloids can adsorb to the interface between two immiscible fluids which results in the stabilisation of emulsions. Examples of polymer solutions near interfaces include systems such as protein adsorption on membranes and responsive polymer layer formation in organic electronics. Below, you will find projects where we investigated the behaviour of molecules, polymer colloids, and solutions at the liquid-liquid or liquid-solid interfaces.
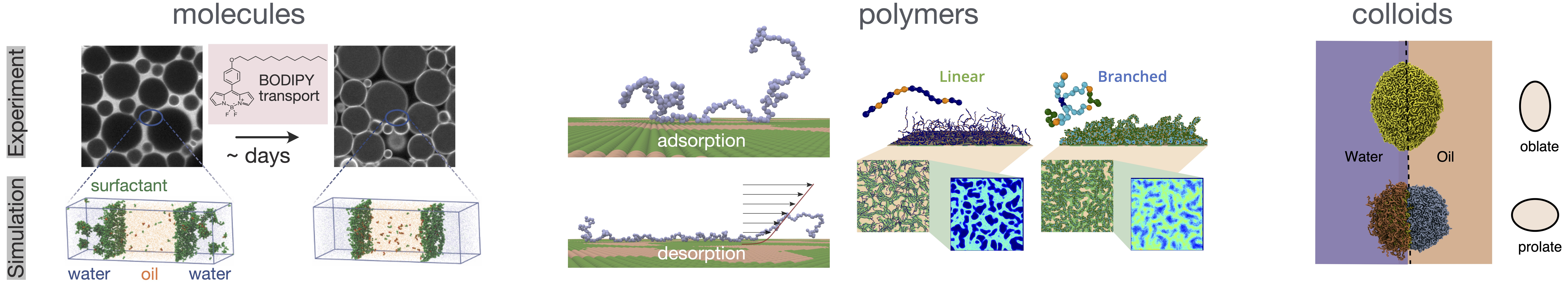
Figure 1: Soft matter systems at interfaces: (left) lipophilic molecules (BODIPY-C12) in oil-in-water emulsions, (middle) polymers of varied architecture near heterogeneous substrates, (right) polymer colloids at the water-oil interface.
In biomedical applications, emulsions often act as carriers to deliver water-insoluble drugs to a specific target. Together with the experimental group from the University of Amsterdam, we investigated the diffusion of a lipophilic fluorescent molecule in oil-in-water emulsions (Bittermann et al., 2023). The transport of these molecules varied with the droplet size, surfactant concentration, and type of surfactant employed. Interestingly, the latter can significantly affect the diffusion of the lipophilic molecules across the oil-water interfaces, where the diffusion time scale can vary from minutes to days. Such a discrepancy could not be simply captured by the hydrophilic-lipophilic balance of surfactants. To gain a molecular understanding of this diffusion process, I developed a coarse-grained description of the system relying on the experimental information. Our calculations revealed that interactions between the head group of a surfactant and a fluorescent part of the lipophilic molecule are key in determining the diffusion of the latter.
In collaboration with L'Oréal Research and Innovation, we examined the formation of adsorbed polymer layers and their subsequent desorption near chemically heterogeneous substrates, which mimic the surface of human hair, as shown in Fig. 1 (middle) (Morozova et al., 2021) . We developed a method in which the experimental images of the substrate are used to obtain information about the surface properties. We probed the behavior of a flexible homopolymer, a semiflexible, linear polysaccharide, and a bottle-brush copolymer. The latter two macromolecules were inspired by cosmetic formulations. We find that polymer architecture plays a key role in the type of the adsorbed structure formed, with bottle-brush macromolecules offering more even surface coverage and exhibiting higher stability against the external flow - an important characteristic in developing hair-care products (Adroher-Benitez et al., 2023).
Polymer colloids with structural complexity, including amphiphilic Janus colloids with two distinct surface domains, e.g., one hydrophobic and one hydrophilic, are promising candidates in many applications. Such Janus colloids can adsorb to the interface between two immiscible fluids and act as emulsion stabilisers similar to surfactant molecules, offering overall higher system stability. We probed amphiphilic Janus and core-shell colloids as well as single-polymer particles (homoparticle) as surface-active agents using coarse-grained molecular dynamics simulations (Morozova & Nikoubashman, 2019). To quantify the performance of the selected particles, we computed the interfacial tension of systems with colloids and a system containing only two fluids, as a reference. Unexpectedly, the reduction of the interfacial tension for systems containing either the Janus particle or homoparticle is almost identical. We rationalize this outcome by looking at the surface area covered by a colloid. Homoparticles offer the largest surface coverage of the liquid-liquid interface due to their oblate ellipsoidal shape, while amphiphilic Janus colloids orient their solvophobic/solvophilic domains toward the corresponding liquids, resulting in a prolate ellipsoidal shape, hence, the lower surface coverage. It should be noted that such changes in colloid shapes upon adsorption at the interface are only possible due to the rubbery nature of the polymers employed.
References
2023
- Surface-mediated molecular transport of a lipophilic fluorescent probe in polydisperse oil-in-water emulsionsLangmuir, 2023
- Effect of polymer architecture on the adsorption and coating stability on heterogeneous biomimetic surfacesMacromolecules, 2023
2021
- Adsorption and desorption of polymers on bioinspired chemically structured substratesACS Applied Materials & Interfaces, 2021