Disordered Proteins and Polypeptides
Proteins are vital biomacromolecules that play a crucial role in cellular functions. They are represented by linear polymers and built from twenty distinct amino acids defining their sequence. It was believed that a well-defined three-dimensional structure of a protein is required for its functioning, the so-called structure-function paradigm. This viewpoint was held until nearly twenty years ago when numerous proteins that lacked intrinsic folded structure under physiological conditions yet remained functional were recognized. These proteins are either intrinsically disordered (IDP) or contain intrinsically disordered regions (IDRs). IDPs and IDRs are characterized by an ensemble of conformations akin to ordinary polymers and are seen as prime candidates to drive phase transitions in living matter. In particular, the organization of the cellular environment into compartments that lack surrounding membranes, i.e., biomolecular condensates (BC) often involves IDPs and IDRs together with nucleic acids. Such spatiotemporal localization of biomacromolecules can facilitate chemical reactions and assembly processes and protect them from harmful environments. Conversely, the alternation in the formation-dissolution cycle of BCs is linked to pathological aggregations such as cancer, infectious and neurodegenerative diseases.
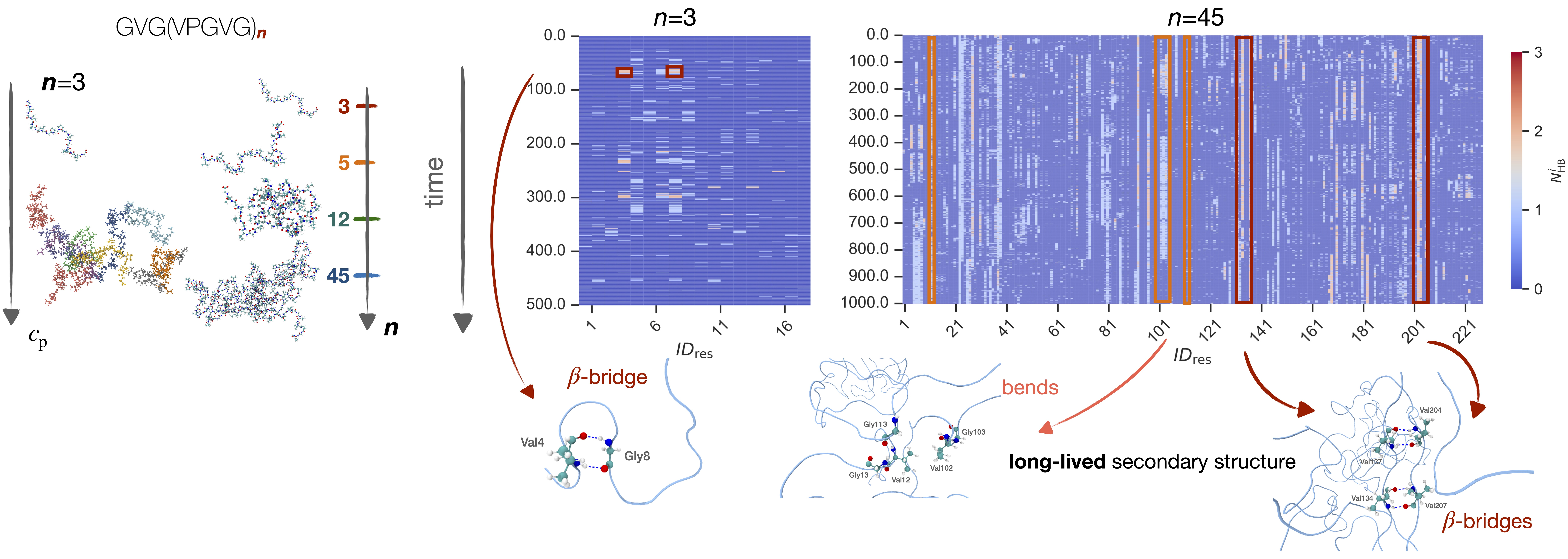
Figure 1: Elastin-like polypeptides studied as a function of temperature, protein concentration, and chain length.
My recent research has focused on two families of IDPs: elastin-like polypeptides (ELPs) and beta-caseins. The former is a synthetic polypeptide derived from the extracellular protein - elastin and is seen as a candidate for therapeutic applications thanks to its biocompatibility. ELPs typically exhibit a lower critical solution temperature phase behaviour characterised by an expanded-to-collapsed conformational change of a polypeptide. In this context, I investigate how polypeptide concentration, temperature, and chain length affect structural and dynamic properties using an all-atom description (Morozova et al., 2023). Most interestingly, we find that despite the ELPs prefer compact states at higher temperatures, short sequences always remain in an extended coil-like structure, while the longer ones favor compact configurations. The former engages in multiple short-lived intrapeptide hydrogen bonds, retaining their dynamic, liquid-like properties, while the latter forms long-lived (hundreds of nanoseconds) intra-peptide hydrogen bonds, attributed to ordered secondary structure motifs such as bridges, hydrogen-bonded turns, and bends (see Fig. 1). Therefore, our work demonstrates the importance of the sequence length as a modulator of conformational properties at a single chain and explains the change in material properties of elastin condensates where short chains form liquid-like aggregates, while longer ELPs form solid-like ones (Morozova et al., 2024).
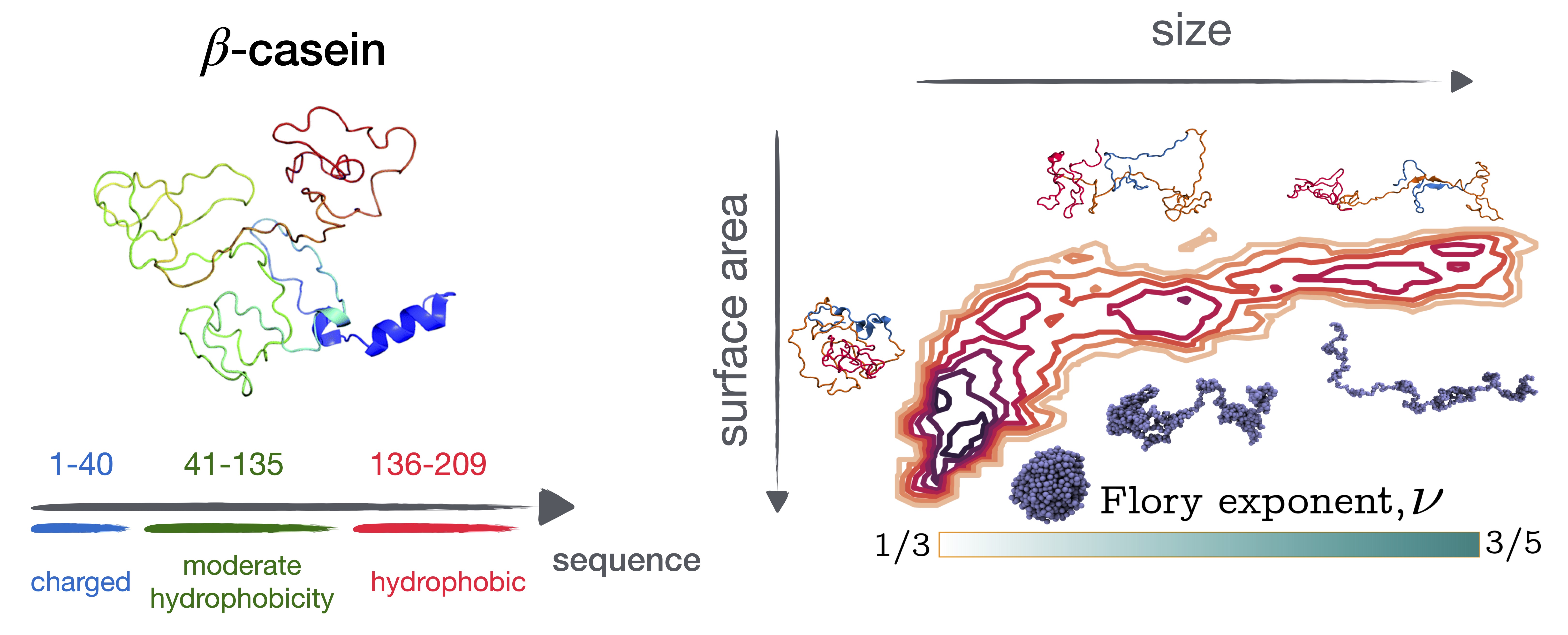
Figure 2: Atomistic structure and the free energy surface of a beta-casein at 300 K.
Together with experimental groups from Grenoble and Tubingen, we investigate beta-caseins - IDPs that are commercially available and assemble in a variety of structures. The structure of a single-casein chain is shown in Fig. 2 (left). Here we wish to address the burning question of how high conformational heterogeneity of these proteins affects the dynamics of their assemblies from the residue level up to the size of aggregates combining numerical and scattering approaches. Using all-atom modeling coupled with advanced sampling methods, we reconstruct their free energy landscape. We find that these IDPs populate both compact and extended states, thus behaving as different polymers in different regions of the conformational space (Chakraborty et al., 2025). We are further complementing this work with experimental results. Stay tuned!
References
2025
- Intrinsically disordered proteins can behave as different polymers across their conformational ensembleThe Journal of Physical Chemistry B, 2025
2024
- Sequence length controls coil-to-globule transition in elastin-like polypeptidesThe Journal of Physical Chemistry Letters, 2024
2023
- Structural and Dynamical Properties of Elastin-Like Peptides near Their Lower Critical Solution TemperatureBiomacromolecules, 2023